The relative tendency of an atom to attract the shared pair of electrons towards itself is called electronegativity.
In
a period from left to right, the value of electronegativity increases
while in a group from top to bottom the value of electronegativity
decreases.
IONIZATION ENERGY
The
ionization energy (IE) is the amount of energy required to remove the
most loosely bound electron, the valence electron, of an isolated
gaseous atom to form a cation.
In a period from left to right, the value of ionization energy increases while in a group from top to bottom the value of ionization energy decreases.
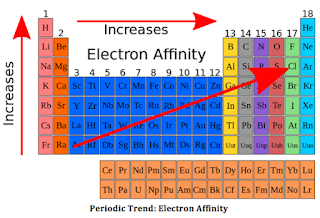
ELECTRON AFFINITY
The
electron affinity of an atom or molecule is defined as the amount of
energy released or spent when an electron is added to a neutral atom or
molecule in the gaseous state to form a negative ion.
In a period from left to right, the value of electron affinity increases while in a group from top to bottom the value of electron affinity decreases.




Post a Comment
Post a Comment